
Individual results may vary.
Bring your beauty
into balance
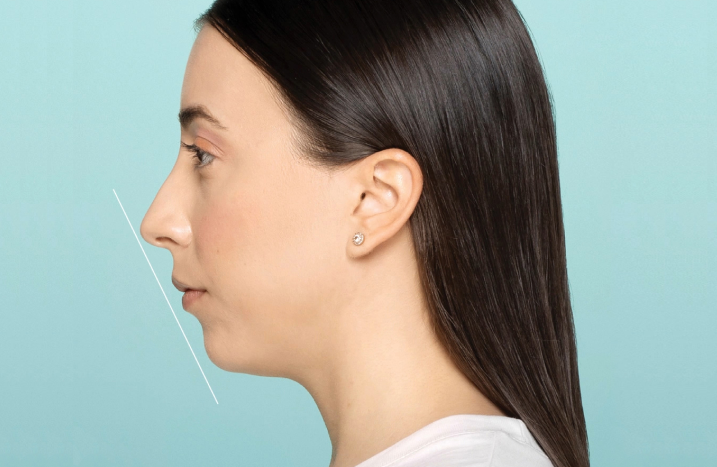
Define your look from every angle
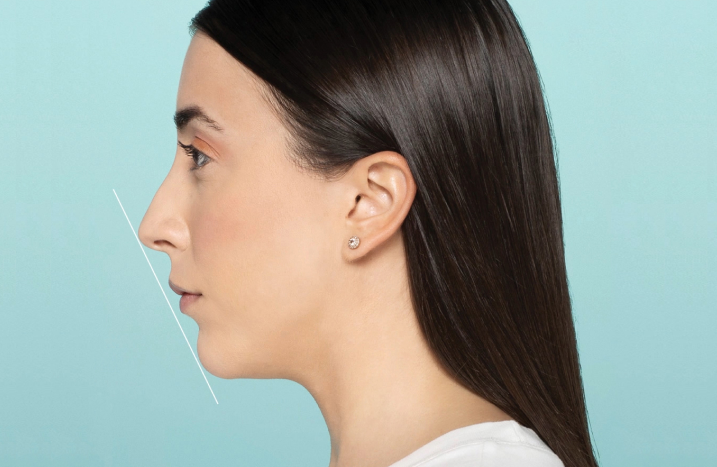
100% of patients had improved facial harmony 9 weeks after lower face treatment with Restylane Defyne3*
Define the chin and soften deep laugh lines2,4

Explore chin filler Before & Afters
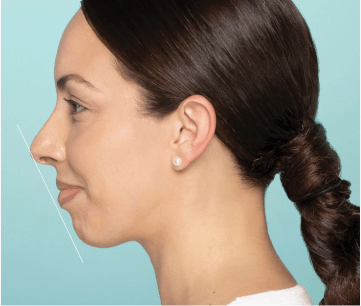

Actual patient. One week after treatment. Individual results may vary.
Chin filler satisfaction
-
98%of patients had improved double chin appearance after treatment with Restylane Defyne.3*
-
95%of patients were satisfied with how symmetrical their face looked after treatment with Restylane Defyne.3*
-
97%of patients reported feeling satisfied with how balanced their face looked after treatment with Restylane Defyne.3*
Frequently Asked Questions
Chin filler is used for the minimally invasive (non-surgical) augmentation of the chin to improve the chin’s projection or shape. Restylane Defyne can be used to reduce the appearance of a double chin, or correct mild-to-moderate chin retrusion, sometimes called a “weak chin.”1,2*
Chin filler can also be used to bring balance to the lower third of the face when a larger-sized lip look is the aesthetic goal. If a “weak chin” is out of proportion with a large lip, the enhancement of the chin will bring these two features into proportion for better harmony of facial features.3
Chin filler has been used in minimally invasive (non-surgical) gender-affirming care as well. The shape of the chin can have a “more traditionally masculine” shape, or a “more traditionally feminine” shape. Filler can help in achieving such looks.4
- Restylane Defyne. Instructions for Use. Galderma Laboratories, L.P., 2023.
- Data on file. 05DF1910 Clinical Study Report. Galderma Laboratories, L.P., 2022.
- Vanaman Wilson, MJ, Jones IT, Butterwick K, Fabi SG. Role of nonsurgical chin augmentation in full face rejuvenation: a review and our experience. Dermatol Surg. 2018;44(7):985-993.
- MacGregor JL, Chang YC. Minimally invasive procedures for gender affirmation. Dermatol Clin. 2020 Apr;38(2):249-260.
For patients with a "weak chin" and a round face, chin filler can be used to augment the chin projection, which will lessen the appearance of roundness of the face, resulting in a more heart- or oval-shaped appearance.1,2
A double chin may emphasize the roundedness of a face’s shape. Restylane Defyne can be used to reduce the appearance of a double chin.1,3*
- Restylane Defyne. Instructions for Use. Galderma Laboratories, L.P., 2023.
- Vanaman Wilson, MJ, Jones IT, Butterwick K, Fabi SG. Role of nonsurgical chin augmentation in full face rejuvenation: a review and our experience. Dermatol Surg. 2018;44(7):985-993.
- Data on file. 05DF1910 Clinical Study Report. Galderma Laboratories, L.P., 2022.
Most Restylane appointments last less than an hour, and results can be seen almost immediately as the added volume begins to smooth away wrinkles.

Join ASPIRE Galderma Rewards to start earning points with qualifying treatments.
Every 100 points = $10 of valuable savings on more of the treatments along your aesthetic journey.
Sign up today and receive a $20 Welcome Reward* for your next treatment.
Find an aesthetic injector
Ready to take the next step? Browse aesthetic specialists near you to book a consultation, discuss your aesthetics goals, and get all your filler questions answered in person.
- Vanaman Wilson, et al. Role of Nonsurgical Chin Augmentation in Full Face Rejuvenation: A Review and Our Experience. Dermatol Surg. 2018;44(7):985-993.
- Restylane Defyne. Instructions for Use. Galderma Laboratories, L.P., 2023.
- Data on file. 05DF1910 Clinical Study Report. Galderma Laboratories, L.P., 2022.
- Data on file. 43USCH1702 Clinical Study Report. Galderma Laboratories, L.P., 2020.
- Restylane® - Instructions for Use
- Restylane-L® - Instructions for Use
- Restylane® Contour - Instructions for Use
- Restylane® Defyne - Instructions for Use
- Restylane® Kysse - Instructions for Use
- Restylane® Lyft - Instructions for Use
- Restylane® Refyne - Instructions for Use
- Restylane® Silk - Instructions for Use
- Restylane® Eyelight - Instructions for Use
- Restylane® - Patient Safety Information
- Restylane-L® - Patient Safety Information
- Restylane® Contour - Patient Safety Information
- Restylane® Defyne - Patient Safety Information
- Restylane® Kysse - Patient Safety Information
- Restylane® Lyft - Patient Safety Information
- Restylane® Refyne - Patient Safety Information
- Restylane® Silk - Patient Safety Information
- Restylane® Eyelight - Patient Safety Information
Important Safety Information
The Restylane® family of products are indicated for patients over the age of 21, and includes Restylane®, Restylane®-L, Restylane® Lyft with Lidocaine, Restylane® Silk, Restylane® Kysse, Restylane® Refyne, Restylane® Defyne, Restylane® Contour, and Restylane® Eyelight.
Restylane and Restylane-L are for mid-to-deep injection into the facial tissue for the correction of moderate-to-severe facial wrinkles and folds, such as nasolabial folds, and for lip enhancement.
Restylane Lyft with Lidocaine is for deep implantation into the facial tissue for the correction of moderate-to-severe facial wrinkles and folds, such as nasolabial folds, and for cheek augmentation and for the correction of age-related midface contour deficiencies. Restylane Lyft with Lidocaine is also indicated for injection into the dorsal hand to correct volume loss. Restylane Lyft with Lidocaine is also indicated for injection into the mid-to-deep dermis (subcutaneous and/or supraperiosteal) for augmentation of the chin region to improve the chin profile in patients with mild-to-moderate chin retrusion.
Restylane Silk is for lip augmentation and for correction of perioral wrinkles.
Restylane Kysse is for lip augmentation and for correction of upper perioral wrinkles.
Restylane Refyne is for mid-to-deep injection into the facial tissue for the correction of moderate-to-severe facial wrinkles and folds, such as nasolabial folds.
Restylane Defyne is for mid-to-deep injection into the facial tissue for the correction of moderate-to-severe, deep facial wrinkles and folds, such as nasolabial folds. Restylane Defyne is also indicated for injection into the mid-to-deep dermis (subcutaneous and/or supraperiosteal) for augmentation of the chin region to improve the chin profile in patients with mild-to-moderate chin retrusion.
Restylane Contour is for cheek augmentation and for the correction of midface contour deficiencies.
Restylane Eyelight is for the improvement of infraorbital hollowing.
Do not use if you have severe allergies that have required in-hospital treatment, are allergic to lidocaine or gram-positive bacterial proteins used to make hyaluronic acid, prone to bleeding, or have a bleeding disorder. The safety of use while pregnant or breastfeeding has not been studied. Tell your doctor if you are planning other cosmetic treatments (such as lasers and chemical peels) as there is a possible risk of inflammation at the injection site.
The most common side effects include swelling, redness, pain, bruising, headache, tenderness, lump formation, itching at the injection site, and impaired hand function. Tell your doctor if you’re taking medications that lower your body’s immune response or affect bleeding, such as aspirin or warfarin; these medications may increase the risk of bruising or bleeding at the gel injection site. Use at the site of skin sores, pimples, rashes, hives, cysts, or infection should be postponed until healing is complete.
Delayed-onset inflammation near the site of dermal filler injections is one of the known adverse events associated with dermal fillers, and cases have been reported to occur at the dermal filler treatment site following viral or bacterial illnesses or infections, vaccinations, or dental procedures. Typically, the reported inflammation was responsive to treatment or resolved on its own. Serious but rare side effects include delayed-onset infections, recurrence of herpetic eruptions, superficial necrosis, and scarring at the injection site. The risk of unintentional injection into a blood vessel is small but can occur and could result in serious complications, which may be permanent including, vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. As with all skin injection procedures, there is a risk of infection.
To learn more about serious but rare side effects and full Important Safety Information, visit www.RestylaneUSA.com.
